Description
What our Physical and Chemical Changes lesson plan includes
Lesson Objectives and Overview: Physical and Chemical Changes introduces students to the difference between a physical change of matter and a chemical one. Students will be able to differentiate between the two types and identify examples of each. They will also learn how to explain these differences to others. This lesson is for students in 4th grade, 5th grade, and 6th grade.
Classroom Procedure
Every lesson plan provides you with a classroom procedure page that outlines a step-by-step guide to follow. You do not have to follow the guide exactly. The guide helps you organize the lesson and details when to hand out worksheets. It also lists information in the yellow box that you might find useful. You will find the lesson objectives, state standards, and number of class sessions the lesson should take to complete in this area. In addition, it describes the supplies you will need as well as what and how you need to prepare beforehand. The activity worksheet requires a number of supplies depending on the experiment students choose to do. You may assign students which experiment they will conduct and provide the necessary materials. The third activity page provides a chart with 12 options for the experiments and the supplies students will need.
Options for Lesson
In the “Options for Lesson” section of the classroom procedure page, you will see some suggestions for additional activities or ideas to add to the lesson if you want to. A few relate to the worksheets. For the activity, you could use demonstrations instead of stations. You could also have students work in groups rather than in pairs. For the homework, students could list chemical and physical changes they observe at home or in the neighborhood. They could create a list throughout the evening or over a weekend and share it with the class. Another option is to invite a chemist to speak to the class about chemical and physical changes. If you want, you could use either the homework or practice page as a quiz or test to determine student comprehension.
Teacher Notes
The teacher notes page provides an extra paragraph of information to help guide the lesson. It suggests you do as many hands-on activities or demonstrations as possible to enhance students’ understanding. If you want, you can teach this lesson in conjunction with others related to chemistry. You can use the blank lines to write down any other ideas or thoughts you have about the topic as you prepare.
PHYSICAL AND CHEMICAL CHANGES LESSON PLAN CONTENT PAGES
Physical and Chemical Properties
The Physical and Chemical Changes lesson plan contains two pages of content. To start off, the lesson reiterates the concept of states of matter. Everything in the world is either a solid, liquid, or gas. All things are matter and take up space. And all matter has physical or chemical properties. The lesson then provides a table that describes physical properties and chemical properties and gives examples of each.
Physical properties are observable using one or more of the five senses. It easy to identify or determine what a substance is without destroying matter. The molecules of these substances do not change. Transparency is a physical property that describes how much light can pass through a substance. Boiling and melting points are likewise physical properties. One more example of physical properties is the strength of a substance—its ability to bend, crack, break, and so on.
Chemical properties indicate how a substance reacts to something else. A substance will change into something new after such a reaction. Unlike with physical properties, the molecules of the substance will change. The flammability of a substance is an example of a chemical property. How a substance reacts with oxygen to produce rust is another example. One more is reactivity with vinegar, which is simply how a substance reacts with vinegar to produce something else.
Students will discover that knowing about the properties of a substance helps them understand the changes that can occur with matter. A physical property of water is that it boils at 212°F, meaning that the water will simply turn into water vapor, a safe gas. Other liquids can become dangerous, however, at certain temperatures because of their chemical properties. Similarly, some metals rust when exposed to water for long periods of time. Others are unaffected by water. Knowing this helps manufacturers decide which metals to use when developing certain products.
Physical and Chemical Changes
Students will now see how knowing the difference between a physical and chemical property can help them identify what type of change occurs with matter. Like the properties of matter, physical and chemical changes in matter have different characteristics to help us identify the changes we observe.
Physical changes include changes in size, shape, color, or phase (solid, liquid, gas). No new substance forms with a physical change—again, the molecules do not change. For example, melting a cube of sugar in coffee is a physical change because the sugar is still sugar. Freezing, boiling, or melting water does not change the water into anything else. Cutting an apple into smaller pieces to make it easier to eat does not change the apple into another substance. Therefore, these are all physical changes.
Chemical changes can affect both the physical and chemical properties of a substance. A chemical change yields a new substance—the molecules do change. As an example, burning a cube of sugar with fire does change the molecules of the sugar into something else. Baking the ingredients of a cake changes many of the molecules due to the high heat. An apple beginning to rot or being digested inside a person’s stomach both change the substance’s molecules. These, then, are all chemical changes.
More Examples
Chemical and physical changes occur all around us every day. If we change the way something looks but don’t make something new, it is a physical change. If we change something into something else, it is a chemical change. When it comes to chemical changes, atoms that make up the molecules change due to a chemical reaction that occurs during that change. The reaction happens usually due to another substance or the addition of heat.
One of the most common chemical reactions is the formation of rust on metal. As mentioned, rust forms on some metals but not on others when the metal is exposed to water for a long period of time. If a person leaves their bike in the rain, it may begin to rust. This is because exposing iron to oxygen gas in the air results in rust. The iron oxidizes to become iron oxide, which is the chemical name for rust.
There are many, many other examples of both physical and chemical changes. But there is a really easy way to remember the difference between the two types of changes. If the molecules of a substance stay the same, this is a physical change. On the other hand, a chemical change occurs when the molecules of a substance change as well.
PHYSICAL AND CHEMICAL CHANGES LESSON PLAN WORKSHEETS
The Physical and Chemical Changes lesson plan includes three worksheets: an activity worksheet, a practice worksheet, and a homework assignment. Each one will help students solidify their grasp of the material they learned throughout the lesson. You can refer to the classroom procedure guidelines to know when to hand out each worksheet.
OBSERVATION STATIONS ACTIVITY WORKSHEET
Students will work with a partner for the activity and visit various stations around the classroom. They will observe what happens at each station and write down what they observed in the chart on the worksheet pages. They will need to circle which type of change they observed and figure out why that change happened. The worksheets provide space for 10 stations. However, there are 12 experiments on the station list from which to choose. You are welcome to do all or just some of these experiments. After students complete the activity, you can ask them to share one or more of their responses and observations.
DESCRIPTION MATCH PRACTICE WORKSHEET
The practice worksheet has two sections. For the first section, students will review 15 statements. They will then mark whether or not each statement describes a physical (P) or chemical (C) change. The second section requires students to match descriptions with the correct term from the word bank. There are five descriptions and terms to match.
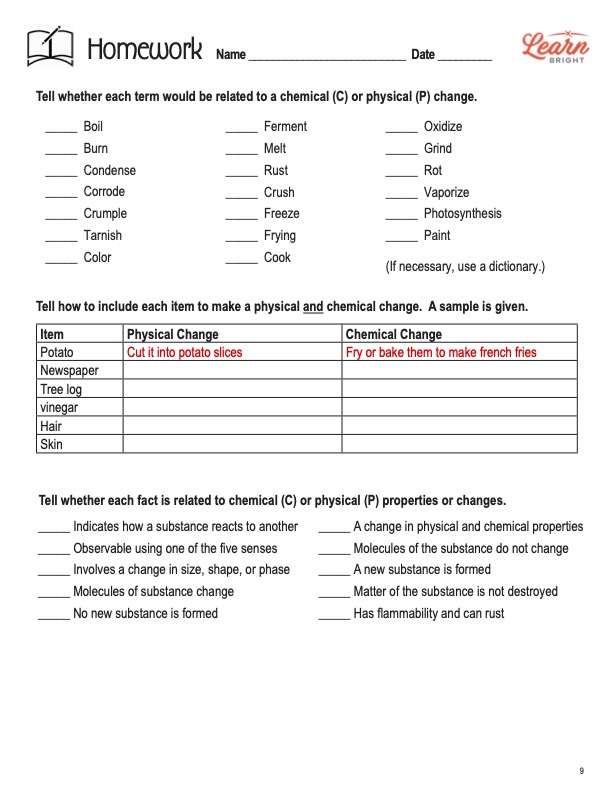
PHYSICAL AND CHEMICAL CHANGES HOMEWORK ASSIGNMENT
Similar to the practice worksheet, the homework assignment has three sections in total. First, students will decide whether each of 20 terms relates to a chemical (C) or physical (P) change. The second section requires students to describe how an item can undergo a physical change and a chemical change. There is a table in which students can fill in the information. The worksheet provides an example that students can use for reference. Finally, students will mark whether each of 10 facts relates to chemical (C) or physical (P) properties or changes.
Worksheet Answer Keys
The lesson plan document provides answer keys for the worksheets. There may be some variation in responses on the homework page. For the most part, however, students’ answers should reflect those of the answer keys. Correct answers are in red to make it easier to compare them with student’s work. If you choose to administer the lesson pages to your students via PDF, you will need to save a new file that omits these pages. Otherwise, you can simply print out the applicable pages and keep these as reference for yourself when grading assignments.