Description
What our Atoms, Molecules, and Matter lesson plan includes
Lesson Objectives and Overview: Atoms, Molecules, and Matter teaches students about various compounds as they relate to matter. Students will discover what these terms are and how they build upon each other. They will learn the differences among these substances and be able to explain them to others. This lesson is for students in 4th grade, 5th grade, and 6th grade.
Classroom Procedure
Every lesson plan provides you with a classroom procedure page that outlines a step-by-step guide to follow. You do not have to follow the guide exactly. The guide helps you organize the lesson and details when to hand out worksheets. It also lists information in the yellow box that you might find useful. You will find the lesson objectives, state standards, and number of class sessions the lesson should take to complete in this area. In addition, it describes the supplies you will need as well as what and how you need to prepare beforehand. This lesson requires quite a few supplies and prep work. Review the “Student Supplies” and “Prepare Ahead of Time” sections on the classroom procedure page to determine which supplies you need and what you need to do before starting the lesson.
Options for Lesson
There are a number of suggestions in the “Options for Lesson” section that you could incorporate into the lesson if you want to or have extra time. Pre-teaching some of the basic ideas of molecular theory is a great place to start. One option is to introduce the lesson with a video about atoms. Check out the Learn Bright video! There is also a lesson on our site, Big Bang Theory, that could be a great lesson to teach in conjunction with this one. Another option is to steer students to various sites on the internet about molecules, compounds, etc., to allow them to further explores these concepts.
Teacher Notes
The paragraph on this page provides a little more information or guidance on what to expect from the lesson. It suggests that you could benefit from teaching this lesson in conjunction with others on states of matter or chemicals. You can use the blank lines to record any thoughts you ideas you have as you prepare.
ATOMS, MOLECULES, AND MATTER LESSON PLAN CONTENT PAGES
Atoms, Molecules, and Matter
The Atoms, Molecules, and Matter lesson plan contains three content pages. Take a quick look around. What do you see? It may not have occurred to you, but everything you see, feel, or smell is made of tiny particles. When these super-tiny particles bond together in different patterns, they form either solids, liquids, or gases. With a mug of hot chocolate, you can see all three properties at once! The mug is a solid, the hot chocolate is liquid, and the steam is a gas.
All these things have weight and take up space. And anything that has weight and takes up space is called matter—even things you can’t see, like air. People, water, food, toys, and everything else are made of matter. So if everything is made of matter, what is matter made of? Great question!
The tiny particles that compose matter are called atoms. Most scientists think atoms are the product of the big bang theory, a theory of how the universe began. The “big bang” happened 13.8 billion years ago, when the universe started as a
tiny, dense fireball that exploded, creating all matter.
You may think that the discovery of atoms is a recent find. But you would
be wrong. A Greek philosopher was the first known person who proposed the idea that everything is made from super-tiny particles that we can’t see. Around 400 BCE—more than 2,400 years ago—Democritus coined the word atomos, which translates to mean indivisible or uncuttable. He hypothesized that unseen small particles combined to form different materials. Amazingly, before the modern world of atomic theory began with John Dalton in the 1800s, Democritus accurately described atoms. What are atoms, and where do they come from?
What Is an Atom?
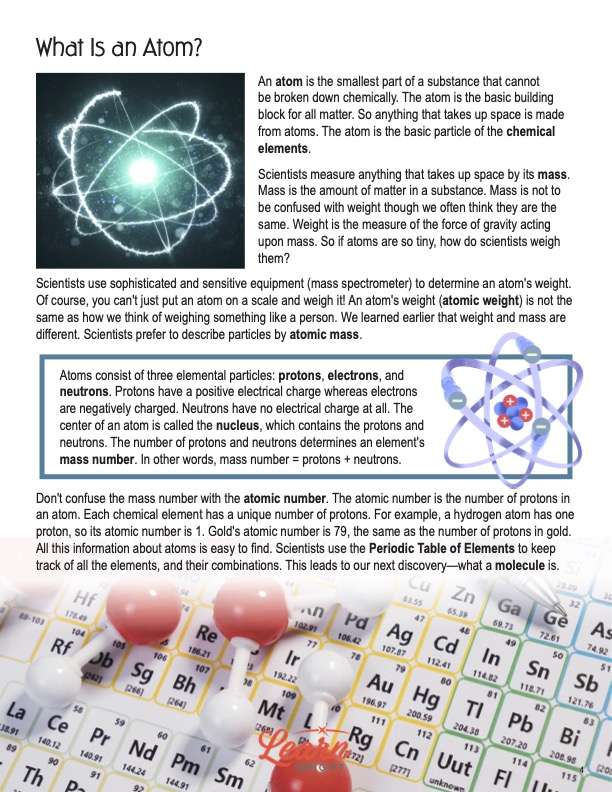
An atom is the smallest part of a substance that cannot be broken down chemically. The atom is the basic building block for all matter. So anything that takes up space is made from atoms. The atom is the basic particle of the chemical elements. Scientists measure anything that takes up space by its mass. Mass is the amount of matter in a substance. Mass is not to be confused with weight though we often think they are the same. Weight is the measure of the force of gravity acting upon mass. So if atoms are so tiny, how do scientists weigh them?
Scientists use sophisticated and sensitive equipment (mass spectrometer) to determine an atom’s weight. Of course, you can’t just put an atom on a scale and weigh it! An atom’s weight (atomic weight) is not the same as how we think of weighing something like a person. We learned earlier that weight and mass are different. Scientists prefer to describe particles by atomic mass.
Atoms consist of three elemental particles: protons, electrons, and neutrons. Protons have a positive electrical charge whereas electrons are negatively charged. Neutrons have no electrical charge at all. The center of an atom is called the nucleus, which contains the protons and neutrons. The number of protons and neutrons determines an element’s mass number. In other words, mass number = protons + neutrons.
Don’t confuse the mass number with the atomic number. The atomic number is the number of protons in an atom. Each chemical element has a unique number of protons. For example, a hydrogen atom has one proton, so its atomic number is 1. Gold’s atomic number is 79, the same as the number of protons in gold. All this information about atoms is easy to find. Scientists use the Periodic Table of Elements to keep track of all the elements, and their combinations. This leads to our next discovery—what a molecule is.
What Is a Molecule?
A molecule forms when two or more atoms bond together. These can be atoms of the same element, like oxygen, but they might also contain two different elements. Every element has unique properties. When they combine, new substances form. And these new substances have their own properties.
When two or more elements or atoms combine to form a substance, we can also call that molecule a compound, if those atoms are not the same element. You may have heard of something called compound words (foot + ball = football) or compound sentences in Language Arts. A similar principle applies to scientific compounds. There are more than 115 elements, and scientists discover new ones periodically. (Again, take a look at the Periodic Table!) But there are countless numbers of molecules and compounds. Let’s look at an example you already know—water.
Water is formed when two atoms of hydrogen bond with one atom of oxygen, which are both gases. When the atoms bond, they create the liquid H2O or water. Now think of a plastic water bottle. Many plastic bottles are made of a chemical called polyethylene terephthalate, or PET for short. The chemical elements that form this compound are carbon, hydrogen, and oxygen. The chemical formula is C10H8O4 (10 carbon atoms + 8 hydrogen atoms + 4 oxygen atoms). Obviously, it takes more work than a few chemicals to make an actual water bottle. But the example illustrates well what happens when elements combine.
And while we are on the subject of water, what makes a liquid a liquid and not a solid or a gas? The molecular particles of a liquid are in motion, though we cannot see them individually. Depending on the substance, an object’s atoms or molecules are either closely compacted (solid), less compacted (liquid), or far apart (gas). The table below explains the properties or traits that make a solid, a liquid, and a gas different.
ATOMS, MOLECULES, AND MATTER LESSON PLAN WORKSHEETS
The Atoms, Molecules, and Matter lesson plan includes three worksheets: an activity worksheet, a practice worksheet, and a homework assignment. Each one will help students solidify their grasp of the material they learned throughout the lesson. You can refer to the classroom procedure guidelines to know when to hand out each worksheet.
MOLECULE MODELS ACTIVITY WORKSHEET
For the activity, students will create models of a molecule (or multiple molecules if you want). The worksheet provides a list of seven molecules for students to choose from. After they pick a molecule and create a model of it using the supplies you provide, they will explain what that compound (molecule) is used for.
CHEMICAL NAMES PRACTICE WORKSHEET
The practice worksheet asks students to write the chemical name for various everyday products like bleach and sugar. Students will need access to the internet to research each product. There are six products to research. When they finish, students can complete the extra practice at the bottom and draw a model of one of the products from the list.
ATOMS, MOLECULES, AND MATTER HOMEWORK ASSIGNMENT
The homework assignment has two sections. First, students will label the four parts of an atom using the terms in the word bank. Then they will answer four questions based on what they learned throughout the lesson.
Worksheet Answer Keys
The last two pages of the lesson plan PDF are answer keys for the practice and homework worksheets. Correct responses are in red to make it easy to compare them with your students’ work. If you choose to administer the lesson pages to your students via PDF, you will need to save a new file that omits these pages. Otherwise, you can simply print out the applicable pages and keep these as reference for yourself when grading the assignment.