Description
What our Acids and Bases lesson plan includes
Lesson Objectives and Overview: Acids and Bases explores acidic and basic substances and how to measure their pH levels. Students will learn the traits of both an acid and a base. They will be able to explain how the two substances differ. They will also learn what pH means and how it helps scientists measure the hydrogen levels of many substances. This lesson is for students in 5th grade and 6th grade.
Classroom Procedure
Every lesson plan provides you with a classroom procedure page that outlines a step-by-step guide to follow. You do not have to follow the guide exactly. The guide helps you organize the lesson and details when to hand out worksheets. It also lists information in the yellow box that you might find useful. You will find the lesson objectives, state standards, and number of class sessions the lesson should take to complete in this area. In addition, it describes the supplies you will need as well as what and how you need to prepare beforehand. You will need a number of supplies for this lesson—white construction paper, scissors, glue, and colored pencils. If you can, try to obtain litmus paper and a variety of liquids to test their pH values.
Options for Lesson
The “Options for Lesson” section on the classroom procedure page provides a number of extra suggestions for additional activities or ways to alter different parts of the lesson. One suggestion is to get litmus paper and about 10 to 12 substances for students to test and identify as either acids or bases. Another idea is to assign the practice worksheet before the activity rather than after. Students could also bring their favorite drink to class and test whether it is basic or acidic according to its pH level. Another option is to invite a chemist to the class to discuss more about acids and bases with the students.
Teacher Notes
The teacher notes page includes a paragraph that provides a little more information about the lesson or more ideas. It suggests obtaining litmus paper if possible as doing so will greatly enhance the lesson plan and students’ learning about acids and bases. You can use the blank lines on the page to write down any other ideas or thoughts you have prior to the lesson.
ACIDS AND BASES LESSON PLAN CONTENT PAGES
The Acids and Bases lesson plan contains three pages of content. The first page explains why all drinks and some foods have a specific taste. Some are more sour while others are on the bitter side. Examples of sour foods or liquids include buttermilk, lemon juice, and some candies. Other things taste bitter. The foods and drinks that have a sour taste are acidic, and the ones with a bitter taste are more basic.
Acids and Bases
The lesson provides lists containing facts about acids and bases along with examples of each. Students will learn that the word acid comes from a Latin word that means sour—acere. Some acids are natural, meaning they exist in nature. People often drink liquids that contain these acids. An acid is a molecule that splits apart in water and releases hydrogen ions.
The stomach contains hydrochloric acid, which it uses to digest food and kill disease-causing germs. Batteries of all kinds also contain a type of acid. That includes the batteries you put in toys or remote controls as well as the ones that go inside a real car. Many drinkable liquids also contain acids, like lemon juice, orange juice, and tomato juice.
Bases, on the other hand, have a bitter taste and a soap-like texture. They just happen to feel soapy when rubbing between the fingers. These substances also occur naturally. A base is a molecule that splits apart in water and releases hydroxide ions, which reduces the number of hydrogen ions.
Examples of bases include soapy water, milk, bleach, and Milk of Magnesia. In the body, the pancreas contains a basic substance that helps with digestion. People use bases in household cleaning products and crop fertilizing.
The Indicators
The first person to define these two types of substances was a chemist named Svante Arrhenius in 1887. It would be impossible to determine whether a substance is acidic or basic by tasting every liquid in nature. It would also be dangerous, after all. Instead, there is a special type of substance scientists use to determine whether a liquid is acidic or basic in nature. This special substance is called an indicator.
There are multiple indicators that help scientists with this task. Indicators change color depending on whether the substance is an acid or a base. Three naturally occurring indicators include litmus, turmeric, and China rose. If someone dips any one of these into a liquid, the liquid will change color, which indicates its base or acid level.
Students will discover that the indicator people most commonly use is litmus. Its natural color is purple. When an acidic solution touches it, it will turn red. On the other hand, it will turn blue if someone dips the litmus into a basic solution. Litmus comes from organisms called lichens, which come from both fungi and algae. In addition, litmus can come in either a paper form or as a solution.
pH Levels
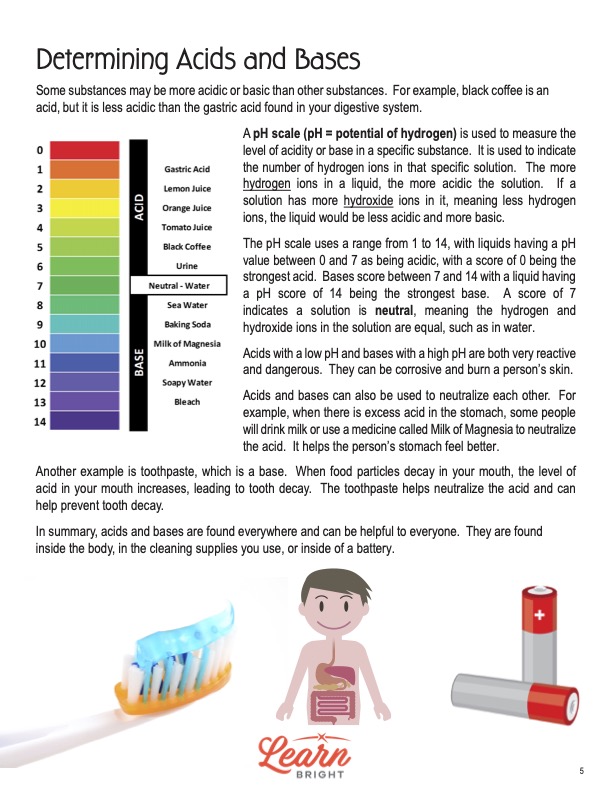
The final page of the lesson discusses pH and how this helps determine the a substance’s acidic or basic level. The lesson shows the pH spectrum that all substances fall on, ranging from 0 to 14. The most acidic substances have a low pH, while the most basic substances have a high pH. This means that while two solutions are acidic, one might be more acidic than the other. For instance, black coffee is acidic, and so is the gastric acid in the digestive system. Black coffee, however, is less acidic than gastric acid and thus has a higher pH level.
A pH scale, then, measures how basic or acidic a solution is. It stands for potential of hydrogen, meaning that it measures how many hydrogen ions are in a specific solution. The more hydrogen ions, the more acidic a solution. The more hydroxide ions, the more basic a solution. Liquids that have a pH between 0 and 7 are acidic, and those with 0 are the strongest acids. Bases have pH scores between 7 and 14, with the score of 14 indicating the strongest base. A score of 7 indicates that a solution is neutral, meaning that there is a balance between both the hydrogen and hydroxide ions. Pure water is a neutral substance with a pH of 7.
Acids with a low pH and bases with a high pH are highly dangerous and very reactive. In fact, they could corrode or burn a person’s skin. But people can use the two types to neutralize each other. When a person’s stomach has excess acid, for example, they could drink milk or use a medicine called Milk of Magnesia to neutralize the acid and make their stomach feel better. Students will be interested to learn that toothpaste actually does something similar! Food particles decay in the mouth and increase the acidity levels, leading to tooth decay. Toothpaste helps neutralize the acid and thus prevent tooth decay.
ACIDS AND BASES LESSON PLAN WORKSHEETS
The Acids and Bases lesson plan includes three worksheets: an activity worksheet, a practice worksheet, and a homework assignment. Each worksheet will help reinforce students’ comprehension of the concepts and material they learned throughout the lesson. You can refer to the guide on the classroom procedure page which outlines when to hand out the worksheets.
ACIDIC TO BASIC ACTIVITY WORKSHEET
For the activity, students will cut out 12 pictures of different substances. Using what they learned during the lesson, they will have to order them from most acidic to most basic. They will glue the images onto a piece of construction paper that shows the pH scale.
ACIDS AND BASES PRACTICE WORKSHEET
There are two parts of the practice worksheet. The first part requires students to match statements to the correct term. There are 15 statements and terms to match up. The second section requires them to compare two liquids to each other. There are 10 pairs of liquids to compare in this section.
WHAT DID YOU LEARN HOMEWORK ASSIGNMENT
Students must answer 20 questions that relate to the information they learned throughout the lesson. If you want to allow students to use the content pages for reference, you may do so. You may, however, wish to test their memory of the lesson material instead.
Worksheet Answer Keys
The final pages of the lesson document are answer keys for the worksheets. The activity answer key displays where each liquid should go and orders them from most acidic to most basic. (In the bases section, you will follow from left to right and top to bottom.) The answers on the practice and homework worksheets are in red. For the most part, students’ responses should match exactly. However, a few questions on the homework assignment may include some variation due to the nature of the question. If you choose to administer the lesson pages to your students via PDF, you will need to save a new file that omits these pages. Otherwise, you can simply print out the applicable pages and keep these as reference for yourself when grading assignments.